Abundances
We all know that some chemical elements like
iron
are more abundant
than others like
gold.
Here you can find more information.
Abundances in Earth's crust
Cosmic Abundances
The Chemical Elements
Here you can find information on all the known chemical elements.
Information on different isotopes of chemical elements can be found
here
.
Periodic table of the chemical elements
Click in picture to continue.
View image
The Periodic Table Songs
Please, skip the advertisements.
http://www.youtube.com/watch?v=zUDDiWtFtEM
and a slower version:
http://www.youtube.com/watch?v=-I7l8TgtuLQ
Elements listed by name and by atomic number
|
Name
|
Symbol
|
number
|
|
Name
|
Symbol
|
number
|
|
Actinium
|
Ac
|
89
|
|
Hydrogen
|
H
|
1
|
|
Aluminum
|
Al
|
13
|
|
Helium
|
He
|
2
|
|
Americium
|
Am
|
95
|
|
Lithium
|
Li
|
3
|
|
Antimony
|
Sb
|
51
|
|
Beryllium
|
Be
|
4
|
|
Argon
|
Ar
|
18
|
|
Boron
|
B
|
5
|
|
Arsenic
|
As
|
33
|
|
Carbon
|
C
|
6
|
|
Astatine
|
At
|
85
|
|
Nitrogen
|
N
|
7
|
|
Barium
|
Ba
|
56
|
|
Oxygen
|
O
|
8
|
|
Berkelium
|
Bk
|
97
|
|
Fluorine
|
F
|
9
|
|
Beryllium
|
Be
|
4
|
|
Neon
|
Ne
|
10
|
|
Bismuth
|
Bi
|
83
|
|
Sodium
|
Na
|
11
|
|
Boron
|
B
|
5
|
|
Magnesium
|
Mg
|
12
|
|
Bromine
|
Br
|
35
|
|
Aluminum
|
Al
|
13
|
|
Cadmium
|
Cd
|
48
|
|
Silicon
|
Si
|
14
|
|
Calcium
|
Ca
|
20
|
|
Phosphorus
|
P
|
15
|
|
Californium
|
Cf
|
98
|
|
Sulfur
|
S
|
16
|
|
Carbon
|
C
|
6
|
|
Chlorine
|
Cl
|
17
|
|
Cerium
|
Ce
|
58
|
|
Argon
|
Ar
|
18
|
|
Cesium
|
Cs
|
55
|
|
Potassium
|
K
|
19
|
|
Chlorine
|
Cl
|
17
|
|
Calcium
|
Ca
|
20
|
|
Chromium
|
Cr
|
24
|
|
Scandium
|
Sc
|
21
|
|
Cobalt
|
Co
|
27
|
|
Titanium
|
Ti
|
22
|
|
Copper (Cuprum)
|
Cu
|
29
|
|
Vanadium
|
V
|
23
|
|
Curium
|
Cm
|
96
|
|
Chromium
|
Cr
|
24
|
|
Dysprosium
|
Dy
|
66
|
|
Manganese
|
Mn
|
25
|
|
Einsteinium
|
Es
|
99
|
|
Iron (Ferrum)
|
Fe
|
26
|
|
Erbium
|
Er
|
68
|
|
Cobalt
|
Co
|
27
|
|
Europium
|
Eu
|
63
|
|
Nickel
|
Ni
|
28
|
|
Fermium
|
Fm
|
100
|
|
Copper (Cuprum)
|
Cu
|
29
|
|
Fluorine
|
F
|
9
|
|
Zinc
|
Zn
|
30
|
|
Francium
|
Fr
|
87
|
|
Gallium
|
Ga
|
31
|
|
Gadolinium
|
Gd
|
64
|
|
Germanium
|
Ge
|
32
|
|
Gallium
|
Ga
|
31
|
|
Arsenic
|
As
|
33
|
|
Germanium
|
Ge
|
32
|
|
Selenium
|
Se
|
34
|
|
Gold (Aurum)
|
Au
|
79
|
|
Bromine
|
Br
|
35
|
|
Hafnium
|
Hf
|
72
|
|
Krypton
|
Kr
|
36
|
|
Helium
|
He
|
2
|
|
Rubidium
|
Rb
|
37
|
|
Holmium
|
Ho
|
67
|
|
Strontium
|
Sr
|
38
|
|
Hydrogen
|
H
|
1
|
|
Yttrium
|
Y
|
39
|
|
Indium
|
In
|
49
|
|
Zirconium
|
Zr
|
40
|
|
lodine
|
I
|
53
|
|
Niobium
|
Nb
|
41
|
|
Iridium
|
Ir
|
77
|
|
Molybdenum
|
Mo
|
42
|
|
Iron (Ferrum)
|
Fe
|
26
|
|
Technetium
|
Tc
|
43
|
|
Krypton
|
Kr
|
36
|
|
Ruthenium
|
Ru
|
44
|
|
Lanthanum
|
La
|
57
|
|
Rhodium
|
Rh
|
45
|
|
Lead (Plumbum)
|
Pb
|
82
|
|
Palladium
|
Pd
|
46
|
|
Lithium
|
Li
|
3
|
|
Silver (Argentum)
|
Ag
|
47
|
|
Lutetium
|
Lu
|
71
|
|
Cadmium
|
Cd
|
48
|
|
Magnesium
|
Mg
|
12
|
|
Indium
|
In
|
49
|
|
Manganese
|
Mn
|
25
|
|
Tin (Stannum)
|
Sn
|
50
|
|
Mendelevium
|
Md
|
101
|
|
Antimony
|
Sb
|
51
|
|
Mercury
|
Hg
|
80
|
|
Tellurium
|
Te
|
52
|
|
Molybdenum
|
Mo
|
42
|
|
lodine
|
I
|
53
|
|
Neodymium
|
Nd
|
60
|
|
Xenon
|
Xe
|
54
|
|
Neon
|
Ne
|
10
|
|
Cesium
|
Cs
|
55
|
|
Neptunium
|
Np
|
93
|
|
Barium
|
Ba
|
56
|
|
Nickel
|
Ni
|
28
|
|
Lanthanum
|
La
|
57
|
|
Niobium
|
Nb
|
41
|
|
Cerium
|
Ce
|
58
|
|
Nitrogen
|
N
|
7
|
|
Praseodymium
|
Pr
|
59
|
|
Nobelium
|
No
|
102
|
|
Neodymium
|
Nd
|
60
|
|
Osmium
|
Os
|
76
|
|
Promethium
|
Pm
|
61
|
|
Oxygen
|
O
|
8
|
|
Samarium
|
Sm
|
62
|
|
Palladium
|
Pd
|
46
|
|
Europium
|
Eu
|
63
|
|
Phosphorus
|
P
|
15
|
|
Gadolinium
|
Gd
|
64
|
|
Platinum
|
Pt
|
78
|
|
Terbium
|
Tb
|
65
|
|
Plutonium
|
Pu
|
94
|
|
Dysprosium
|
Dy
|
66
|
|
Polonium
|
Po
|
84
|
|
Holmium
|
Ho
|
67
|
|
Potassium
|
K
|
19
|
|
Erbium
|
Er
|
68
|
|
Praseodymium
|
Pr
|
59
|
|
Thulium
|
Tm
|
69
|
|
Promethium
|
Pm
|
61
|
|
Ytterbium
|
Yb
|
70
|
|
Protactinium
|
Pa
|
91
|
|
Lutetium
|
Lu
|
71
|
|
Radium
|
Ra
|
88
|
|
Hafnium
|
Hf
|
72
|
|
Radon
|
Rn
|
86
|
|
Tantalum
|
Ta
|
73
|
|
Rhenium
|
Re
|
75
|
|
Tungsten (Wolfram)
|
W
|
74
|
|
Rhodium
|
Rh
|
45
|
|
Rhenium
|
Re
|
75
|
|
Rubidium
|
Rb
|
37
|
|
Osmium
|
Os
|
76
|
|
Ruthenium
|
Ru
|
44
|
|
Iridium
|
Ir
|
77
|
|
Samarium
|
Sm
|
62
|
|
Platinum
|
Pt
|
78
|
|
Scandium
|
Sc
|
21
|
|
Gold (Aurum)
|
Au
|
79
|
|
Selenium
|
Se
|
34
|
|
Mercury
|
Hg
|
80
|
|
Silicon
|
Si
|
14
|
|
Thallium
|
Tl
|
81
|
|
Silver (Argentum)
|
Ag
|
47
|
|
Lead (Plumbum)
|
Pb
|
82
|
|
Sodium
|
Na
|
11
|
|
Bismuth
|
Bi
|
83
|
|
Strontium
|
Sr
|
38
|
|
Polonium
|
Po
|
84
|
|
Sulfur
|
S
|
16
|
|
Astatine
|
At
|
85
|
|
Tantalum
|
Ta
|
73
|
|
Radon
|
Rn
|
86
|
|
Technetium
|
Tc
|
43
|
|
Francium
|
Fr
|
87
|
|
Tellurium
|
Te
|
52
|
|
Radium
|
Ra
|
88
|
|
Terbium
|
Tb
|
65
|
|
Actinium
|
Ac
|
89
|
|
Thallium
|
Tl
|
81
|
|
Thorium
|
Th
|
90
|
|
Thorium
|
Th
|
90
|
|
Protactinium
|
Pa
|
91
|
|
Thulium
|
Tm
|
69
|
|
Uranium
|
U
|
92
|
|
Tin (Stannum)
|
Sn
|
50
|
|
Neptunium
|
Np
|
93
|
|
Titanium
|
Ti
|
22
|
|
Plutonium
|
Pu
|
94
|
|
Tungsten (Wolfram)
|
W
|
74
|
|
Americium
|
Am
|
95
|
|
Uranium
|
U
|
92
|
|
Curium
|
Cm
|
96
|
|
Vanadium
|
V
|
23
|
|
Berkelium
|
Bk
|
97
|
|
Xenon
|
Xe
|
54
|
|
Californium
|
Cf
|
98
|
|
Ytterbium
|
Yb
|
70
|
|
Einsteinium
|
Es
|
99
|
|
Yttrium
|
Y
|
39
|
|
Fermium
|
Fm
|
100
|
|
Zinc
|
Zn
|
30
|
|
Mendelevium
|
Md
|
101
|
|
Zirconium
|
Zr
|
40
|
|
Nobelium
|
No
|
102
|
top
Abundances in Earth's crust
Abundance (atom fraction) of the chemical elements in Earth's
upper continental crust
as a function of atomic number. The rarest elements in the crust (shown in yellow) are the most dense. They were further rarefied in the crust by being
siderophile
(iron-loving) elements, in the Goldschmidt classification of elements.
Siderophiles were depleted by being relocated into the Earth's core.
Their abundance in
meteoroid
materials is relatively higher.
Additionally, tellurium and selenium have been depleted from the crust due to formation of
volatile
hydrides.
Click in picture to continue.
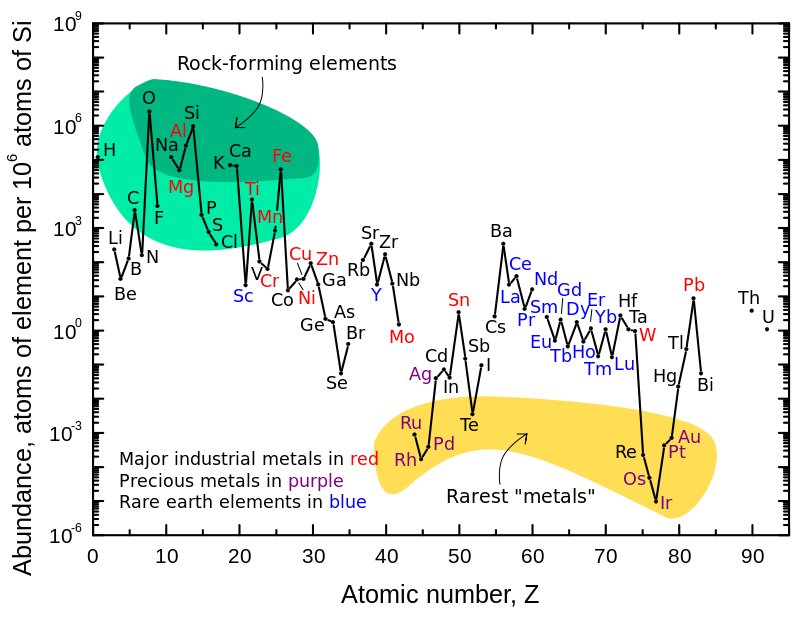
View image
top
Cosmic Abundances
From the human perspective only the abundances of the chemical elements
in the Earth's crust and atmosphere is relevant, but it is not typical
for the Earth as a whole or for the Solar system.
Click in picture to continue.
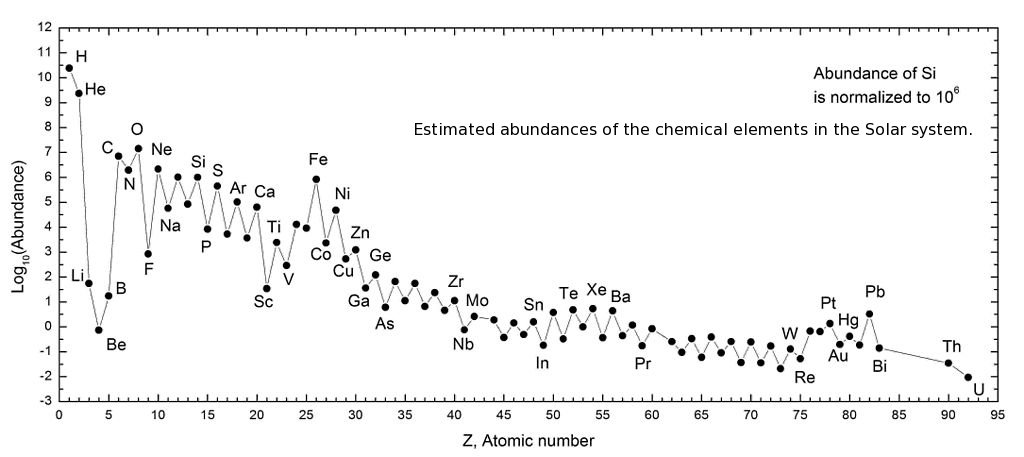
View image
Moreover the cosmic abundances evolved during the history of the universe
by nuclear reactions mainly in the cores of heavy stars and during supernova
explosions.
Click in picture to continue.
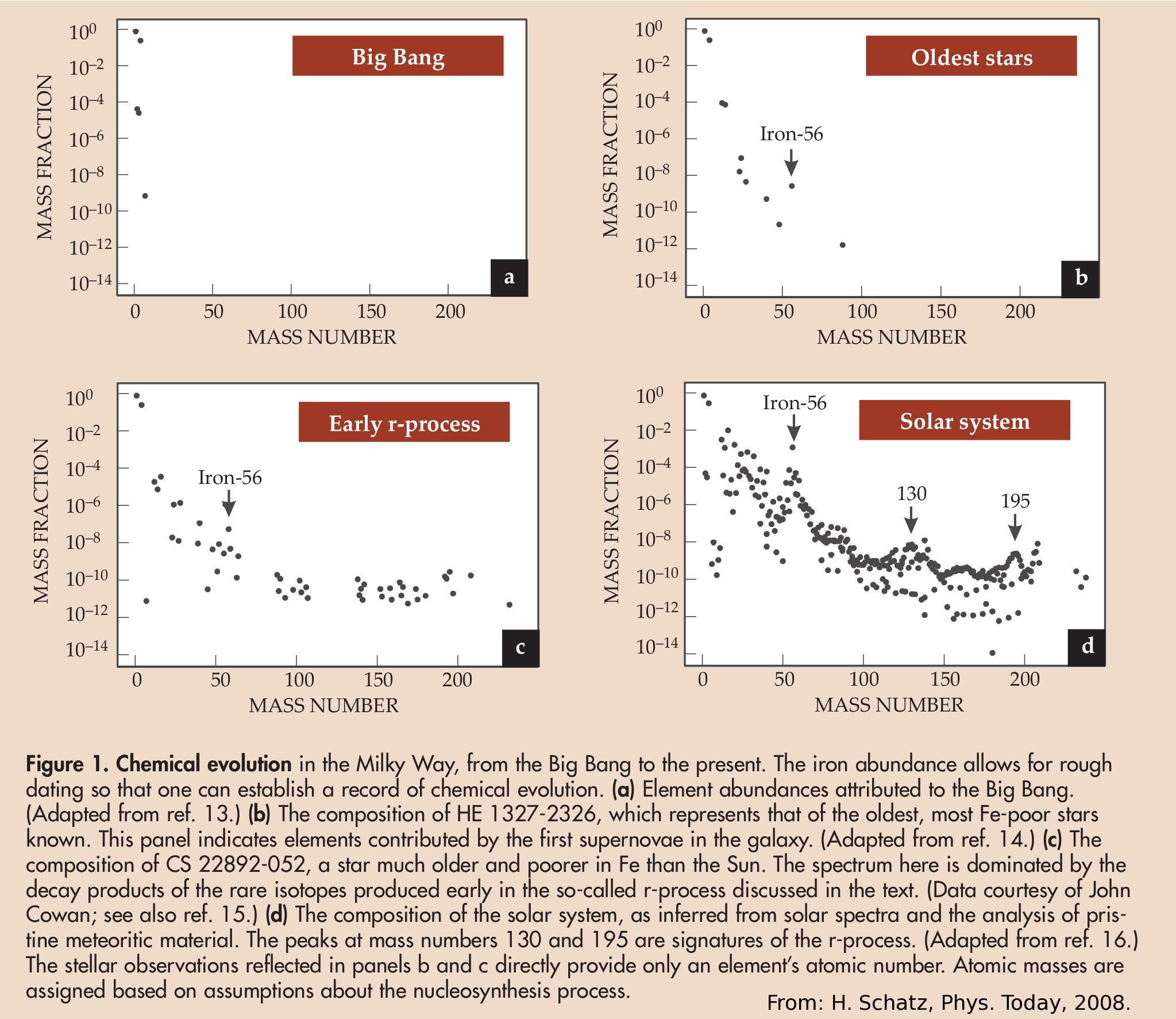
View image
Read the complete article by H. Schatz in Physics Today
here
(PDF)
.
top




