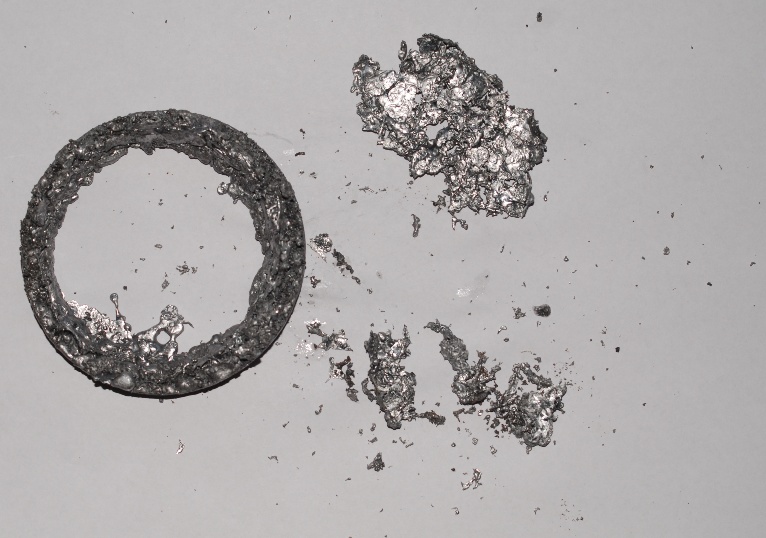home
Nederlands
Thermite on ice
Introduction
Orthography
In the British literature the mixture of aluminium and iron oxide is
called
Thermit
(
Metal and Thermit Corporation
). However in
Chambers Dictionary of Science and Technology (London, 1971)
we find the lemma
thermite
and the term
Thermit welding
.
Chambers Technical Dictionary (London, 1953)
gives the same information.
The Concise Oxford Dictionary 10th ed. (Oxford, 1999)
gives as lemma
thermite
(also)
thermit
.
The general term for reduction of metaloxides by burning aluminium powder,
ignited with magnesium ribbon, is the
aluminothermic process
.
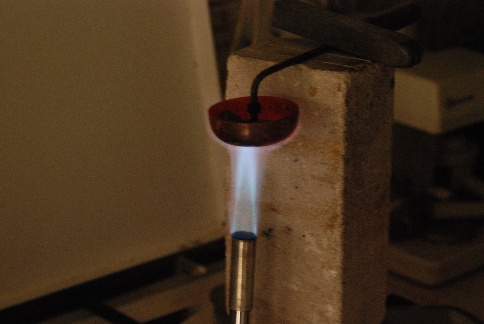
What is thermit[e]?


Other ways to connect rails
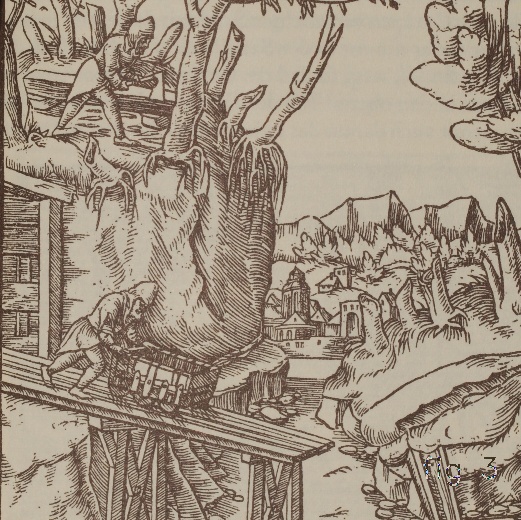 Carts on wooden rails were already in use in the 16th C (fig 3).
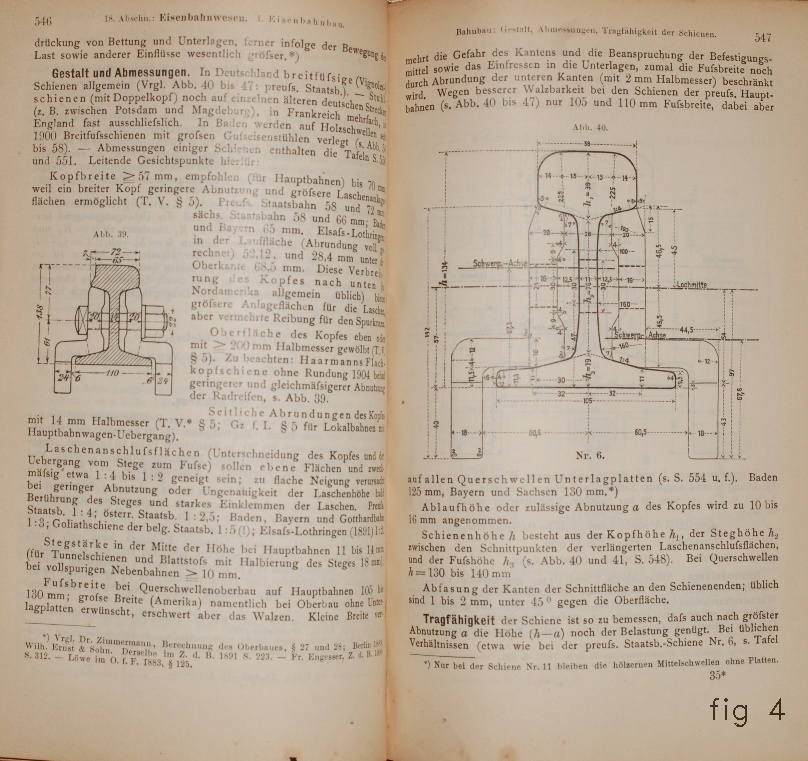
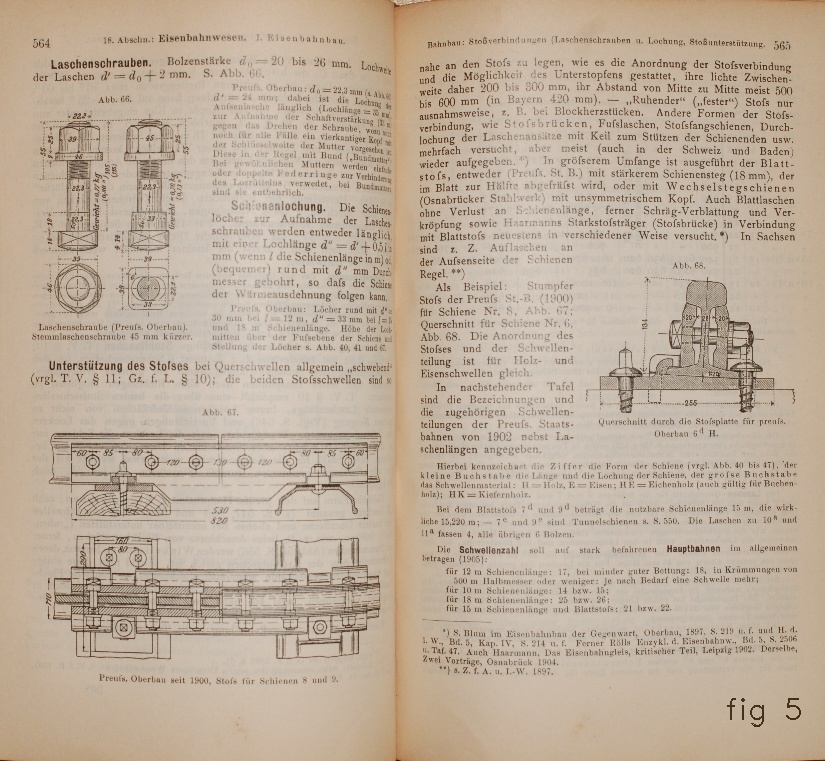
The traditional way to connect stretches of rails for modern railways
is by what in French is called
sabots
(fig 4,5) (from there
saboteurs
, who remove these things to derail trains).
Carts on wooden rails were already in use in the 16th C (fig 3).
The traditional way to connect stretches of rails for modern railways
is by what in French is called
sabots
(fig 4,5) (from there
saboteurs
, who remove these things to derail trains).
The problem is thermal expansion, which is 0.0000105/K for steel.
So with a temperature range of -20°C to +30°C we have to deal with
1 cm length change per 20 m of rails. If the track is curved (tramways!)
this is not so much of a problem and rails welded by the thermit
process or otherwise can be used right away. Other solutions are to allow stresses
to build up in welded rails or use special sections were larger thermal
length change can be compensated.
Some 100000 Chinese engineers (*) are currently building a high speed
rail network in that country. May be they have some time left to answer
further questions on this subject.
References
fig 1,2
Newnes "Complete Engineer" Series - Volume 18,
E.Molloy, Welding and metal cutting (London, 1945).
fig 3
Agricola, De Re Metallica (Basel, 1556; Faksimiledruck Düsseldorf, 1977).
fig 4,5
Des Ingenieurs Taschenbuch 19e Aufl. Abteilung II (Berlin, 1905).
(*)
BBC World Service, 25 january, 2011 - 09:46 GMT "China: Shaking the World" Part 1.
http://www.bbc.co.uk/worldservice/documentaries/2010/05/100513_china_shaking_the_world_part_one.shtml
The Challenge
In
New Scientist 30 Jan 2010
you can read in "The LAST WORD":
-- quote --
THE LAST WORD
MythBusters challenge
New Scientist
has teamed up with Discovery Channel's
MythBusters
to
attempt to solve a mystery. Thermite and ice can make an explosive
combination, so don't try this experiment at home - watch it safely
on the web at
http://www.mythbusters-thermite.notlong.com
. We want
to find out why the explosion happens. Thermite is a mainstay of
pyrotechnics, comprising a mixture of metal and metal oxide powders that
burns at extremely high temperatures in a tightly focused area. Thermite is
not, by itself, explosive, but if you ignite a bucket of thermite on top of
blocks of ice, there is an enormous bang once it has burnt through the
bucket.
MythBusters
presenters Jamie Hyneman and Adam Savage
are seeking a convincing scientific explanation of this violent reaction.
Will The Last Word readers solve the mystery?
-- end of quote --
Different nonsensical suggestions were made, but few or no experiments were done.
This may be due to the lucky difficulty to get hold of thermit (and to
ignite it). I did a simple (if dangerous) experiment and used Occam's razor.
I send the following e-mail to New Scientist.
2010-02-01
To lastword@newscientist.com
Cc mythbusters@m5industries.com
I was rather disappointed by the answers on the MythBusters challenge
about thermite on ice (The Last Word, New Scientist 30 january 2010).
Did nobody do the simple - if dangerous - experiment I did once I read about the question
why thermite and ice can make an explosive combination: pour molten lead on ice?
Pouring molten lead at about 400 °C, a temperature where oxidation is insignificant,
on a slab of ice results in a violent steam explosion which ejects the lead as small droplets.
Do not do this at home without drastic safety precautions !!
There is no need to assume that the explosion which happens when iron molten
by thermite is poured on ice is anything else than a steam explosion which disperses the molten iron.
The spectacular firework is a consequence of the increased surface area of the glowing metal.
The MythBusters are in an ideal position to perform the experimentum cruxis: pour molten gold on ice.
kind regards,
H.J. Schoonhoven
address and contact info
By the way around 1830 aluminium was more exensive than gold.
Pouring lead on ice
There is a large difference between pouring molten lead

in a bucket of water
(a Finnish passtime)


and pouring molten lead on ice.



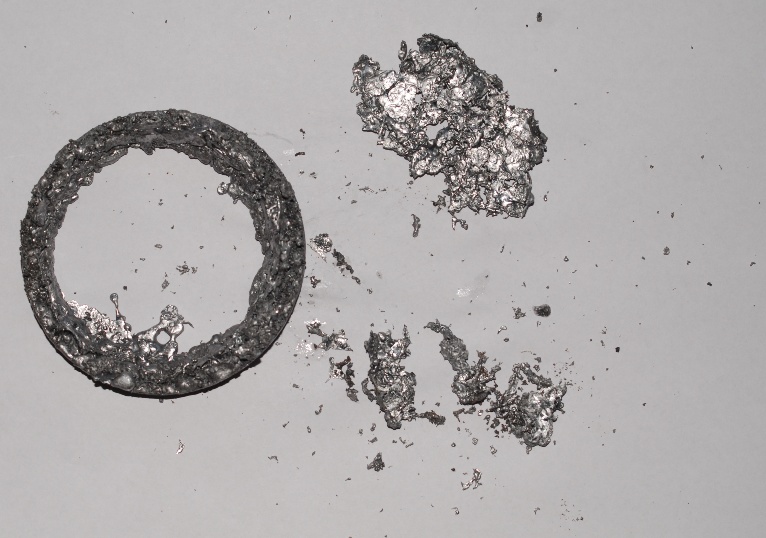
By pooring molten lead in
water
steam is formed under water, the steam
condenses in the cold water and the lead, although fragmented, sinks to the bottom.
By pooring molten lead on
ice
steam is formed
between
the metal and the ice. The still molten metal is ejected. You could hear the bangs of the escaping steam.
For reasons of safety and also to recover the lead I contained it in a
carton box (10cm ø, 32 cm high). In the first experiment I used a tin can
as container. Afterwards the metal covered the inside of the can.
Imagine what happens when you let white hot molten iron from the Thermit
process fall on ice .....
The brightening of the resulting fireball is related to the brightening of a supernova (one exploding star which outshines an entire galaxy).
home (English)
Nederlands


 Carts on wooden rails were already in use in the 16th C (fig 3).
The traditional way to connect stretches of rails for modern railways
is by what in French is called
sabots
(fig 4,5) (from there
saboteurs
, who remove these things to derail trains).
Carts on wooden rails were already in use in the 16th C (fig 3).
The traditional way to connect stretches of rails for modern railways
is by what in French is called
sabots
(fig 4,5) (from there
saboteurs
, who remove these things to derail trains).